The INFINITY™ with ADAPTIS™ Technology Total Ankle System consist of ADAPTIS™ 3D printed, porous metal tibia tray, the option of a chamfered or flat cut ADAPTIS™ talar dome, and the EVERLAST™ Highly Cross-linked polyethylene. All components are available in varying sizes to best match your anatomy.
Key Benefits:
1. Data on file at Wright. Claim based on data from rabbit femur model. It is unknown how these results compare with clinical results in humans.
2. When compared to Wright Medical traditional ankle UHMWPE poly inserts through bench top testing according to ISO 22622. Data on file.
3. Zhang Y, Ahn PB, Fitzpatrick DC, Heiner A, Poggie RA, Brown TD, Interfacial frictional behavior; cancellous bone, cortical bone, and a novel porous tantalum biomaterial.Journal Musculoskeletal Research 1999.
4. Hsu AR, Davis WH, Cohen BE, Jones CP, Ellington JK, Anderson RB. Radiographic Outcomes of Preoperative CT Scan-Derived Patient-Specific Total Ankle Arthroplasty. Foot Ankle Int. May 4 2015.

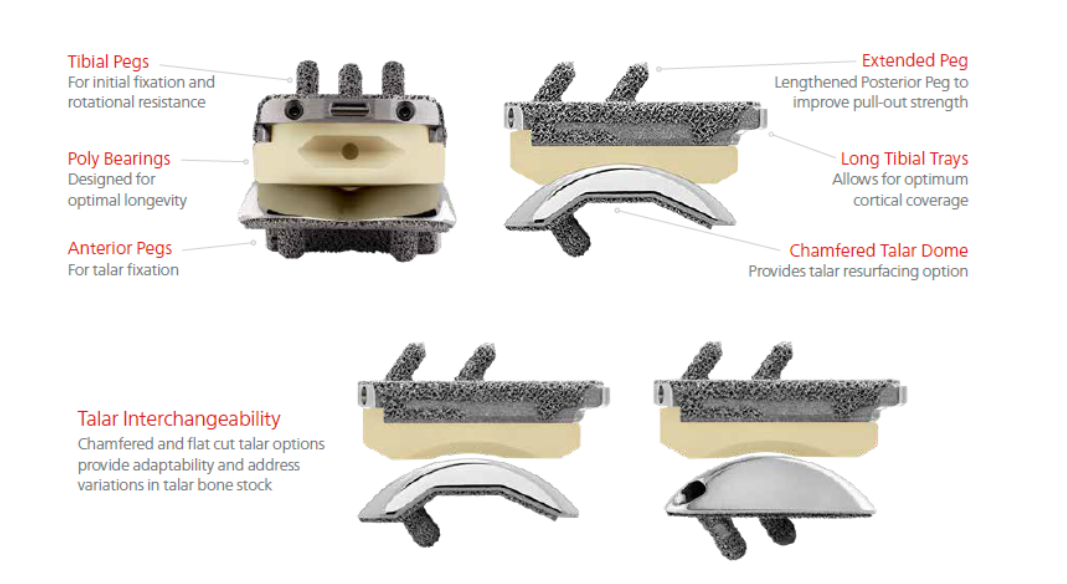
The INFINITY™ tibial tray was designed with a standard resection height for all sizes (1 through 5), as well as the ability to upsize to a “long” AP size tray without requiring additional bone resection.
The tibial tray features 3 fixation pegs with lengths and angles optimized to facilitate anterior delivery into prepared joint space. This design allows the tibial tray to be impacted axially (not anteriorly) to help ensure proper seating and stability. The pegs are press fit into the prepared tibia to provide solid initial fixation.
 EVERLAST™ Highly Cross-linked Polyethylene UHMWPE starts with GUR-1020E, which is Type 1 resin infused with Vitamin E.
EVERLAST™ Highly Cross-linked Polyethylene UHMWPE starts with GUR-1020E, which is Type 1 resin infused with Vitamin E.
Crosslinking improves the wear properties of UHMWPE but can leave free radicals which overtime could cause it to oxidize, degrade, and potentially cause a failure mode known as delamination. The Vitamin E acts as an antioxidant which helps prevent oxidation from the crosslinking without using the traditional thermal treatments such as remelting or annealing which have been shown to weaken the material or not completely eliminate the residual free radicals2.
2. UHMWPE Biomaterials Handbook, 3rd edition.
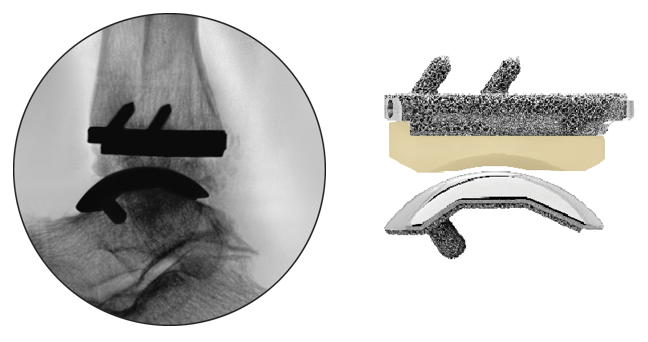
The INFINITY™ prosthesis was purposefully designed without tibial flanges or barrels and without talar dome side walls that would obscure fluoroscopic imaging.
By allowing complete visualization of the implant interface, the surgeon can verify thorough seating of the prosthesis. This important feature also facilitates the visibility of X-rays for post-op follow up.
INFINITY™ with ADAPTIS™ Technology features the same sulcus articular geometry as INBONE™ II. A coronally stable design that may help reduce dependency on surrounding soft tissues.
INFINITY™ with ADAPTIS™ Technology features a low-profile, 3D printed, porous chamfered and flat-cut style option allowing surgeons preference to dictate preparation options.
